The Maillard reaction for non-chemists

Do you know the roaster’s joke?
“Do not talk to the roaster during the Maillard reaction”
Since the Maillard reaction practically always takes place, it means something like: Don’t talk to me, no matter when.
Hardly any other chemical reaction attracts as much attention in the coffee world as the Maillard reaction.
This article is for you if you want to know:
what the two types of browning reactions are for food
which two non-enzymatic reactions are particularly important for us in the coffee sector and how they work
why they are so important for us
The article could end after this paragraph. However, two important questions would then remain unanswered.
Foods turn brown in two ways: one is through the enzymatic browning reaction and the other is through the non-enzymatic browning reaction.
Take a cut apple or a banana. They turn brown through the enzymatic browning reaction. In the so-called polyphenol oxidase, enzymes react with air and convert invisible phenol into brown components that we then see at the cut surface. These brown components are polymers and toxic to bacteria. Voilà, the apple and the banana thus form a protective layer against intruders. So it is a matter of self-defense.
Our coffee, on the other hand, gets its brown color from the other, non-enzymatic browning reaction. This can be divided into three types: the Maillard reaction, caramelization and ascorbic acid oxidation.
During the Maillard reaction, reduced sugars and available amino acids react under the influence of temperature. Caramelization describes the process in which sugars oxidize or break down under the influence of temperature. Ascorbic acid oxidation is still the closest thing to an enzymatic browning reaction, although it requires other enzymes, such as peroxidase.
The article could end here, because now we know which reaction makes coffee brown and what the other types of non-enzymatic browning reactions are.
But we would leave two important questions unanswered, namely:
What happens during the Maillard reaction, what factors influence it and what is the difference to caramelization?
And: Why is this process so important for the coffee world?
Before we get to the “why” and the significance of the Maillard reaction for the world of coffee, let’s take a look at the individual phases of the Maillard reaction, as well as some factors that influence it.
Plus: what exactly is the difference between the Maillard reaction and caramelization?
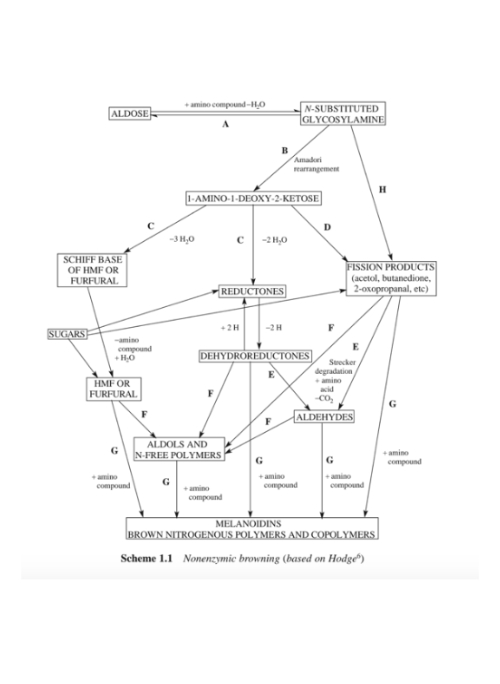
Mr. Hodge has also given thought to the graphical representation of the Maillard reaction.
It shows in a simplified way which reactions occur at which point in time in the Maillard reaction, which breakdown products they form and which components (rather simple sugars or rather amino components) react.
Source graphic: See below in the references number 2.
The 3 phases of the Maillard reaction (explained simply for non-chemists)
In 1953, African-American chemist John E. Hodge divided the Maillard reaction into three phases. These do not have a particularly characteristic name and are still used today as a rough subdivision of this highly complex browning reaction (note: if you are a chemist and have different information, please kindly contact me).
Let’s take a closer look at the three phases of the Maillard reaction according to Hodge.
In the first phase, two reactions take place:
On the one hand, reduced sugar molecules and molecules of the amino acid react to form condensation water and unstable glycosylamines, which are amino sugars or simple sugars.
Also in the first phase, the Amadori rearrangement (aldoses), named after its Italian discoverer Mario Amadori, or the Heynes rearrangement (ketones), named after the German chemist Kurt Heynes, occurs. Which of the two rearrangements takes place depends on the chemical group of the sugars, i.e. whether they are ketones or aldoses.
The rearrangement makes the sugar molecules more stable in their composition. In this first phase, the resulting products are all colorless.
Many other reactions are also taking place, and there is well-documented research on the Amadori rearrangement. For our purposes, we will leave it at that for now.
Let’s move on to the second phase:
Phase two comprises three reactions: sugar dehydration, sugar fragmentation and Strecker degradation.
As we can see, it is again about sugar molecules and their conversion. During sugar dehydration, varying numbers of water molecules are removed from the sugar molecules. This depends on whether the reaction takes place in an acidic, neutral or alkaline environment. In an acidic environment, furfural and hydroxymethylfurfural are formed.
Neither of these components is responsible for the brown color, the melanoidins, in coffee. They are more of a by-product. However, these components are not useless: there are indications that they contribute to the ripening of food, especially in the first eight weeks of its formation.
In a neutral or alkaline environment, reductones are primarily formed. Here, too, sugar molecules lose water, but not as many as in the formation of furfural. Like furfural, reductones contribute to the loss of smell and taste of food during storage. On the positive side, their antioxidant effect and – as with furfural – their contribution to the browning of food should be noted.
During the Maillard reaction, many catalyzing reactions take place. One of these is the retro-aldol reaction or reverse aldol reaction. This is where we really get into organic chemistry. What is important for us to know is that fission products from sugars are formed during this reaction.
But not only sugar molecules change, the amino acids also undergo a conversion. And this takes place during the Strecker degradation, named after its discoverer Adolph Strecker (1822-1871). This process is important for us in the coffee sector, because it produces breakdown products that contribute to the aroma of our coffee.
The resulting aldehyde compounds (pyrazines, pyridines, furans, etc.) contribute to the complexity of the aromas of food.
In this second phase, the resulting products are colorless to yellowish or slightly brownish.
Phase three comprises two reactions
The aldol condensation and the aldehyde-amino condensation. The melanoidins are formed at the end of this and all the preceding reactions.
So we have almost made it through reading about the phases of the Maillard reaction.
The melanoidins are now formed, among other things, by aldehydes, which we know from the Strecker degradation and which are formed in phase two by the so-called aldol condensation and the aldehyde-amino condensation. Melanoidins are heavy protein building blocks and to date it is not possible to identify all the elements that form these melanoidin compounds.
Hodge noted at this stage: “The products of this phase are brownish.”
Let’s summarize
So, that’s it! It wasn’t difficult, although it may have been a bit dry for non-chemists.
Let’s summarize what we now know about the Maillard reaction:
- It is a sequence of chemical reactions that take place both successively and simultaneously.
- Above all, it is a reaction between simple carbohydrates (“sugars”) and proteins (“amino acids”).
- It produces melanoidins and many other aromatic products.
- It is very complex.
- It has a major influence on the formation and breakdown of aromatics in food.
- It does not require enzymes to take place, which is why it is classified as a non-enzymatic browning reaction.
These factors influence the Maillard reaction
Our crux as coffee roasters: every Maillard reaction is a little different. Many different factors influence its course. Let’s take a look at what these factors are and which ones we can control:
- The moisture in the green coffee and the water activity: the amount of water and its activity have a major influence on the Maillard reaction. There is evidence that high water activity hinders the reaction of simple sugars and amino acids.
- The pH value: We have already seen in the second phase of the individual reactions that the Maillard reaction produces different cleavage products depending on whether it takes place in a predominantly acidic or alkaline environment.
- The temperature: a Maillard reaction can take place at low temperatures, but it really picks up speed the higher the temperature rises. There is evidence that the processes and products of the Maillard reaction double with every 10-degree increase in temperature.
We can assess the moisture and water activity in the green coffee and adjust our roast. But strictly speaking, these factors are beyond our control, because it is primarily the producers in the growing country who have a direct influence. Our task in the roasting plant is limited to good storage.
What we can definitely control is the temperature, or rather, the energy.
And then there is the… caramelization phase
Compared to the Maillard reaction, it is quick to explain:
At high temperatures of 160 degrees Celsius or more, sugar begins to melt or break down into its individual components. As the heat continues to rise, water molecules also dissolve and the components of the sugar reform.
The result is caramel-like, brownish components that have a similar aroma (although without the creamy and sweet taste of real caramel with cream).
So caramelization adds color and aroma to our coffee. But something else is created as well: carbon dioxide (CO2). And we all know (at least by hearsay) the event during roasting when the pressure of the carbon dioxide in the bean becomes too great and escapes with a crack: that’s right, that’s the second crack.
To sum up, we note that caramelization
- begins at high temperatures, from 160 degrees Celsius.
- It is also a non-enzymatic browning reaction.
- The by-products of color, aroma and carbon dioxide are formed.
Overview of similarities and differences
Let us briefly survey the similarities and differences between the Maillard reaction and caramelization.
What the two reactions have in common is that they produce flavors and the characteristic color of coffee.
They differ in that they take place at different temperatures: While the Maillard reaction takes place at room temperature (albeit very, very slowly), caramelization requires temperatures above 150 degrees Celsius.
They also differ in the substances they use for the reaction: the Maillard reaction is based on simple sugars and amino acids. Caramelization is based only on simple sugars.
Why should we care about these reactions?
Now that we know that there are enzymatic and non-enzymatic browning reactions in food, and that the Maillard reaction and caramelization are among the non-enzymatic reactions, we come to a very exciting point: Why do we deal with this and why should we really be interested in it if we are coffee roasters?
The answer can be divided into three categories (maybe more will arise, in which case I will update this section):
- Food safety and security
- Aromatics and sensory properties
- Effects of storage
Food safety and security
Furan and acrylamide are by-products that develop during the roasting process. Neither of these is good for your health. However, furan is volatile and there are indications that it simply evaporates during preparation and does not enter the cup. On the other hand, good substances that have an antioxidant effect on the body are also formed. All bitter substances, for example, are among them. If we consider only the Maillard reaction, then there are even further effects on the human body, for example reactions with the body’s own proteins and in connection with diabetes mellitus.
Aromatics and sensory properties
The Maillard reaction and caramelization play a decisive role in the aroma of roasted coffee. It is only through the complex and catalytic reactions that take place during roasting that countless new, aromatically effective compounds are formed from acids, sugars and amino acids. We use sensory methods to assess the quality of a roast and to detect any foreign or off-flavors. We only have to think of the characteristic “body/mouthfeel”, which is due to the presence of melanoidins. This enables us to draw conclusions about the point in the roasting process at which we can intervene to obtain our desired flavor profile.
Effects of storage
By-products of the Maillard reaction cause food to lose its aroma and flavor more quickly. In coffee, this effect often manifests as a process known as “staling”, which refers to stale, flat, stale aromas. The argument of the freshness of roasted coffee is affected here and knowing when we can intervene in the roasting process can be an advantage.
Conclusion
Louis Camille Maillard’s discovery in 1912 and John E. Hodge’s systematic review in 1953 have had a significant impact on the food industry to this day. Research into the Maillard reaction is receiving a new impetus from its effects on the aging process in the human body.
Even if we do not need to understand the detailed chemical processes of this complex reaction (we leave that to chemistry PhD students and look forward to the results), the significance for the world of coffee must be clear to us.
The by-products and results of these non-enzymatic reactions, Maillard and caramelization, have a positive effect, for example, in terms of aroma and antioxidants, and a negative effect, for example, through acrylamide and furan.
As coffee roasters, we can influence our roasts to produce a safe and aromatically pleasing product.
Sources
Liedke, Ralph. 1999. Formation of α-dicarbonyl compounds during the degradation of Amadori rearrangement products. Doctoral thesis. Last accessed on: January 21, 2021
Nurstein, Harry. 2005. The Maillard reaction. Last accessed: January 21, 2021
Rivera, Joseph A.2008. The science of browning reactions. In: The book of roast, p. 161 ff.